In recent years, research on high-efficiency, low-cost water electrolyzers has gained widespread attention, as large-scale hydrogen production and utilization are crucial for enhancing the resilience of renewable electricity generation and transmission systems. Currently, the most common method of hydrogen production is through steam reforming of methane or other hydrocarbons, but this process generates significant carbon dioxide emissions. Therefore, water electrolyzers that generate hydrogen and oxygen via electrochemical water splitting have become a research hotspot.
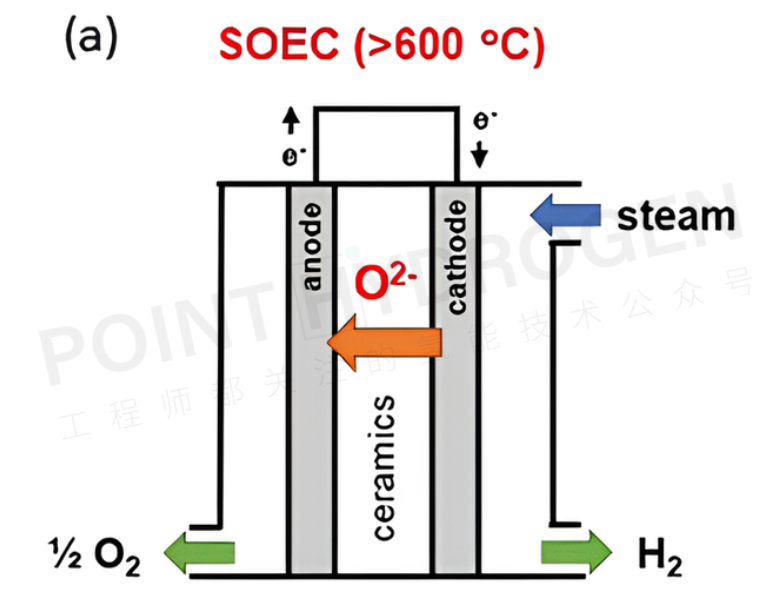
Under high-temperature operating conditions (700–950°C), solid oxide steam electrolyzers (SOECs) have been developed and verified at the laboratory and pilot scale (see Figure 1). The high operating temperature of SOECs allows them to operate at relatively low cell voltages with almost no kinetic limitations, achieving near 100% high heating value (HHV) electrolysis efficiency at a current density of about 1 A/cm². However, high-temperature operation also brings many challenges, such as long startup and shutdown times, rapid degradation due to high-temperature interdiffusion of cell components, and poisoning caused by corrosion products, making SOECs face difficulties in market deployment.
Issues with Alkaline and PEM Electrolyzers
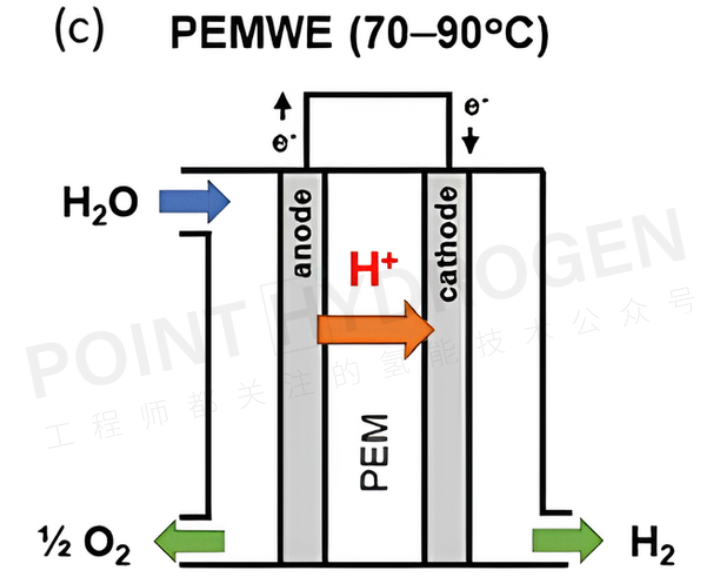
Proton exchange membrane water electrolyzers (PEMWEs) utilize proton exchange membranes (PEM) and ionomers in the electrodes, allowing operation without circulating liquid electrolytes. In this configuration, both the anode and cathode are in direct contact with the non-porous PEM, forming a compact cell arrangement (zero-gap design) (see Figure 3). This design enables PEMWEs to operate at current densities of around 2 A/cm².
Furthermore, the non-porous membrane in PEMWEs supports differential pressure operation, enabling high-pressure hydrogen generation at the cathode and atmospheric pressure oxygen generation at the anode. This reduces the need for secondary mechanical compression for hydrogen storage. Despite these advantages, the high cost of electrocatalysts (such as iridium oxide and platinum), and corrosion-resistant current collectors and bipolar plates used in acidic environments, may become limiting factors for large-scale systems. This is especially true as the stack size increases, and these components significantly contribute to the overall system cost. Both AWEs and PEMWEs are considered mature technologies and have been commercially deployed based on specific application needs.
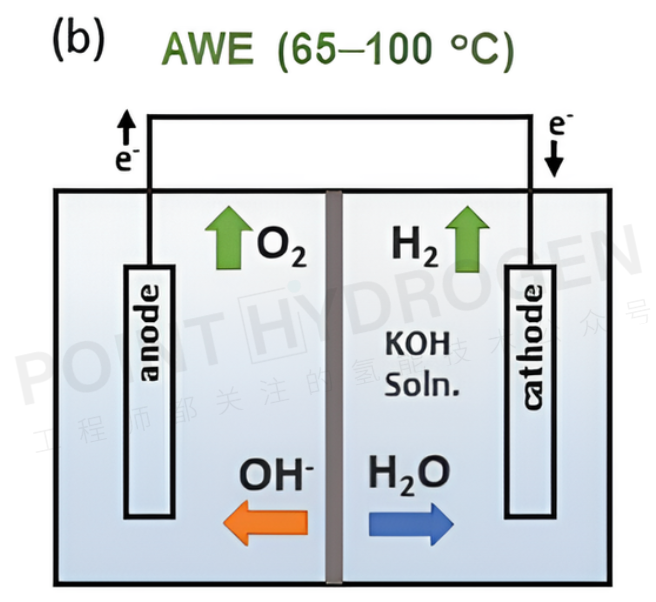
At low-temperature operating conditions (below 100°C), alkaline water electrolyzers (AWEs) are a mature technology. AWEs use an aqueous solution containing potassium hydroxide (KOH) as the liquid electrolyte and are equipped with porous separator membranes (see Figure 2). Extensive research has been reported on the development of platinum group metal (PGM)-free electrocatalysts for hydrogen and oxygen evolution reactions (i.e., hydrogen evolution reaction (HER) and oxygen evolution reaction (OER)). The current research direction focuses on designs such as zero-gap configurations to increase current density or operating pressure. However, AWEs have relatively low hydrogen production rates, typically around 200 mA/cm² at a cell voltage of 1.8 V.
AEM Electrolyzer Operating Principles
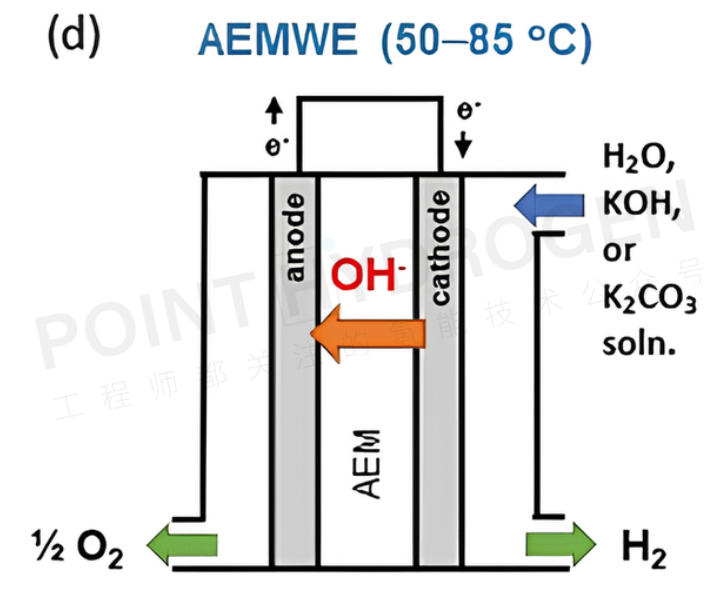
Anion exchange membrane water electrolyzers (AEMWEs) operate in an alkaline environment and can use platinum group metal (PGM)-free catalysts. The anion exchange membrane (AEM) is a non-porous hydrogen-oxide conductive polymer with fixed positively charged functional groups on its main or side chains, enabling zero-gap configurations and differential pressure operation (see Figure 4).
The overall reaction in AEMWEs involves the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Water or alkaline liquid electrolyte circulates through the cathode, where water is reduced to hydrogen and hydroxide ions by adding two electrons (H₂O + 2e⁻ → H₂ + OH⁻). The hydroxide ions diffuse through the AEM to the anode, while electrons are transferred through the external circuit to the cathode. At the anode, hydroxide ions recombine to form oxygen and water, generating two electrons (2OH⁻ → ½O₂ + H₂O + 2e⁻). Hydrogen and oxygen gases form as bubbles at the HER and OER catalyst surfaces. Similar to PEMWEs, the non-porous membrane zero-gap configuration of AEMWEs allows for high-speed hydrogen production and reduces the need for mechanical compression for hydrogen storage.
It is noteworthy that AEMWEs combine the advantages of AWEs (PGM-free catalysts) and PEMWEs (zero-gap configurations and non-porous membranes). Interestingly, unlike PEMWEs, which exclusively use polymer electrolytes, many AEMWEs also employ liquid electrolytes (such as KOH or K₂CO₃ solutions).
Recent modeling studies suggest that adding liquid electrolyte not only reduces the ohmic resistance of the membrane and catalyst layer but also improves reaction kinetics. By adding liquid electrolyte to the cell, the local pH at the catalyst-electrolyte interface increases, creating an additional electrochemical interface. Industrial AEMWEs with nickel-based catalysts in 1 M KOH solution produce hydrogen at a voltage of 2 V and a current density of 1.8 A/cm², achieving performance comparable to conventional PEMWEs at atmospheric pressure. Due to the low cost of catalysts and hardware, as well as the applicable zero-gap configuration and differential pressure operation, AEMWEs are gaining increased interest in hydrogen production.
Durability Challenges of AEM Electrolyzers
The primary technical challenge of AEMWEs (Anion Exchange Membrane Water Electrolyzers) in commercially viable systems is their durability. Durability in AEMWEs generally refers to the lifetime of the device. During the early stages of AEMWE development, measuring durability was relatively easy as the cell lifespan was shorter (less than 500 hours). However, as more durable AEMWEs are developed, measuring their lifespan has become more complicated.
It is important to note that running a cell for over 10,000 hours takes more than a year. Therefore, the durability of AEMWEs is typically assessed by measuring the voltage change rate in long-term tests (100-1000 hours) or by using accelerated stress tests (AST) under accelerated degradation conditions (such as higher operating temperatures and high current densities). However, it should be noted that long-term tests using voltage change rates and lifespan tests under AST conditions may not accurately predict the durability of AEMWEs, as cell lifespan is affected by multiple degradation modes and is often limited by catastrophic failure. Thus, it remains necessary to run the cell continuously under normal operating conditions to obtain its true lifespan.
Although the stack lifetime of commercial proton exchange membrane water electrolyzers (PEMWEs) is close to 20,000 to 60,000 hours, the reported lifetime of most AEMWEs is around 3,000 hours. Furthermore, most AEMWEs are tested under atmospheric pressure conditions.