Although the cost of PEM electrolyzers has been decreasing and AEM technology is gradually advancing, there is no doubt that alkaline water electrolysis still dominates the current market. Alkaline water electrolysis for hydrogen production is a process that uses KOH or NaOH aqueous solution as the electrolyte, and applies direct current (DC) to the electrolyzer. This results in the decomposition of water molecules into hydrogen and oxygen under the influence of the electric field. In this process, hydrogen is produced at the cathode and oxygen at the anode, with the generated hydrogen needing to undergo de-alkalization treatment. This technology has advantages such as simplicity, low cost, and maturity, but its electrolytic efficiency is relatively low, and the equipment's lifespan is significantly affected by frequent start-stop cycles or power variations. Proper control of the important process parameters during water electrolysis can ensure the stability and safety of the production process, while also greatly impacting efficiency and product quality.
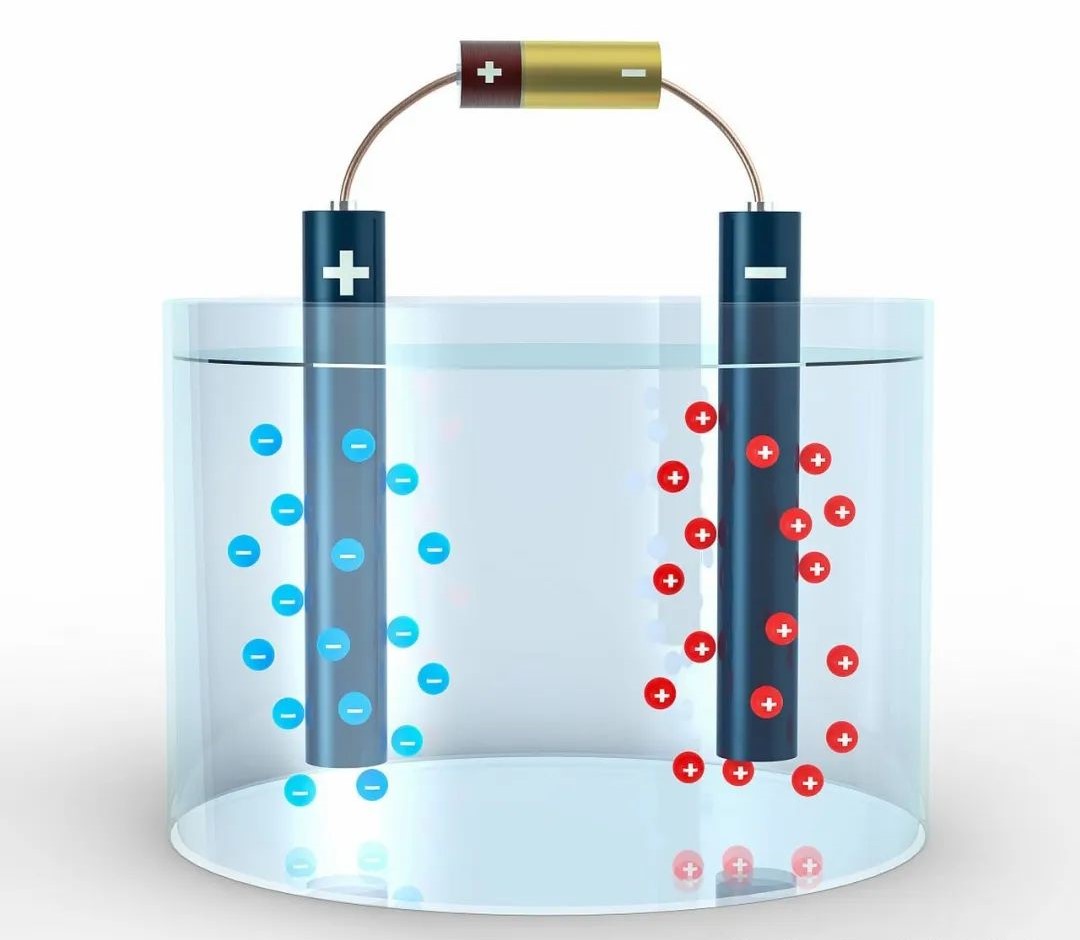
Here are several key indicators in water electrolysis hydrogen production:
Raw water level: Water is the raw material for electrolysis in hydrogen production. Controlling the raw water level within a specified range is essential for the long-term stable operation of the electrolysis system.
Hydrogen production system pressure: The pressure of the hydrogen production system is a critical indicator for normal system operation. Excessive pressure can affect the safe operation of the system.
Purification pressure: The purification pressure is also a key indicator for system operation. Low pressure can reduce the purification efficiency, while high pressure can compromise the safety of the system.
Hydrogen-oxygen level: The hydrogen-oxygen level reflects the pressure difference between the hydrogen and oxygen sides of the system. It must be maintained within a specific range during operation to prevent excessive pressure differences, which could cause hydrogen-oxygen mixing.
Alkaline solution temperature: The alkaline solution temperature influences the small cell voltage of the electrolyzer.
Hydrogen-oxygen tank temperature: The temperature of the tank is critical. High temperatures can affect the seals and membranes of the electrolyzer, while low temperatures can reduce the electrolytic efficiency. Do you know why the temperature on the hydrogen side of the electrolyzer outlet is slightly higher than on the oxygen side?
Alkaline solution circulation rate: The circulation rate of the alkaline solution affects the purity of the gas produced.
Hydrogen in oxygen, oxygen in hydrogen: This is an important performance indicator of the electrolyzer. If it is too high, it can lead to serious production accidents. What factors influence this indicator?
Dew point: This reflects the capability of the purification system.