Proton Exchange Membrane Electrolysis (PEM electrolysis) uses a proton exchange membrane as the electrolyte, where the following chemical reactions occur at the anode and cathode:
Anode:
2H₂O → O₂ + 4H⁺ + 4e⁻
Cathode:
4H⁺ + 4e⁻ → 2H₂
PEM electrolysis is an efficient water electrolysis technology primarily used to split water into hydrogen and oxygen. The PEM electrolysis device consists of an electrolyzer and auxiliary systems, with the core components of the electrolyzer including the membrane electrode, gas diffusion layer, and bipolar plates. The membrane electrode is one of the key components of the proton exchange membrane electrolysis device. The proton exchange membrane (PEM) is coated with catalytic layers on both sides, forming the membrane electrode. The cathode catalyst is typically a platinum-based catalyst, similar to that used in fuel cells, which effectively promotes hydrogen generation. The requirements for the anode catalyst are more stringent due to the strongly oxidative environment at the anode side; the oxygen evolution reaction requires the use of oxidation-resistant and corrosion-resistant catalytic materials. Currently, iridium (Ir), ruthenium (Ru), and their oxides (such as IrO₂ and RuO₂) are the most commonly used anode catalysts, as these materials exhibit excellent stability and catalytic performance, maintaining good electrolysis efficiency at high current densities.
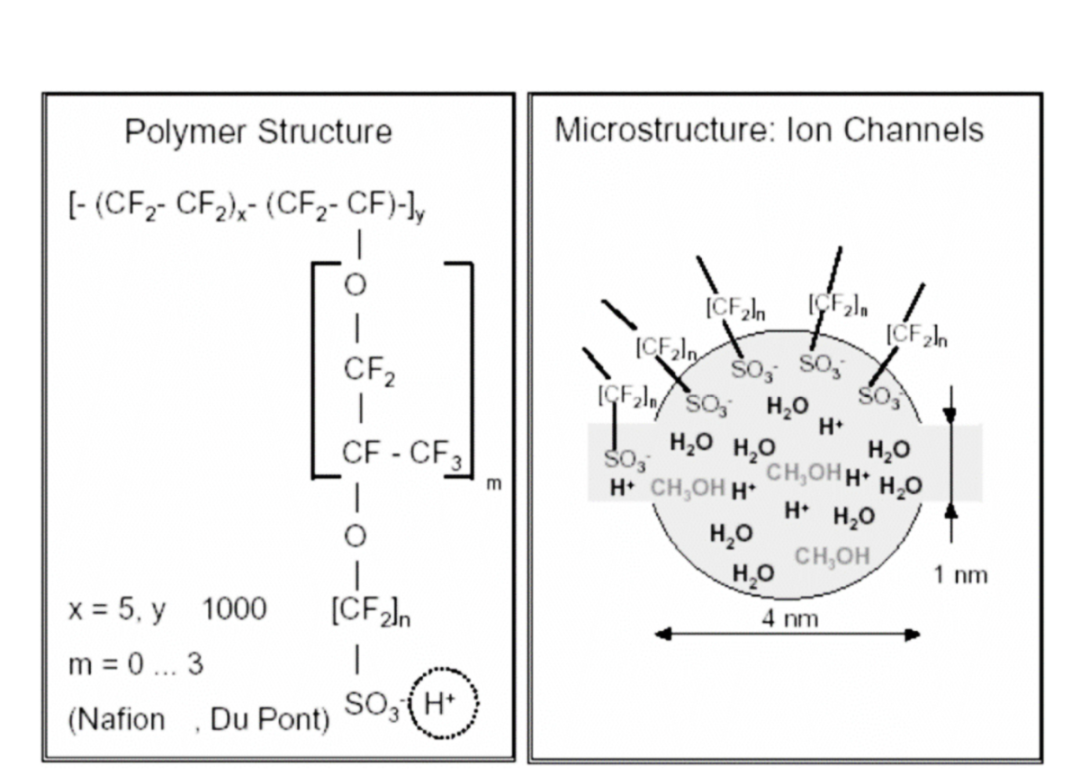
The proton exchange membrane (PEM) plays a critical role in PEM electrolysis devices. Commonly used PEM materials include the Nafion series, such as Nafion 115 and Nafion 117, which have high proton conductivity and chemical stability, effectively isolating gases and conducting protons. Due to the thinness of the proton exchange membrane, its resistance is low, allowing the PEM electrolysis device to withstand high currents and pressures without stringent pressure control on both sides of the membrane. Moreover, PEM electrolysis devices can start and stop quickly, responding rapidly to power adjustments, making them suitable for fluctuating inputs from renewable energy sources.
The Gas Diffusion Layer (GDL) is another important component of PEM electrolysis devices. The GDL is typically made of porous titanium-based materials coated with precious metals, which not only provide good conductivity and mechanical strength but also offer a uniform gas diffusion path, thereby enhancing electrolysis efficiency and gas production.
PEM electrolysis technology has many advantages. First, the high proton conductivity and low resistance of the proton exchange membrane allow PEM electrolyzers to operate at high current densities, increasing hydrogen production. Secondly, the compact structure of PEM electrolysis devices enables high power density, allowing for significant hydrogen production within limited space. Additionally, PEM electrolysis devices can quickly start and stop, adapting to the variability of renewable energy generation, making them particularly suitable for integration with wind and solar energy for green hydrogen production.
However, PEM electrolysis technology also faces some challenges. The first is the cost of catalysts, particularly the expensive precious metals like iridium and ruthenium required for the anode catalyst, which limits large-scale application. Furthermore, the durability and chemical stability of the proton exchange membrane and gas diffusion layer need further research and optimization. With ongoing advancements in materials science and manufacturing technology, it is believed that these issues will gradually be addressed in the future.
In conclusion, PEM electrolysis technology demonstrates significant potential in hydrogen production, especially in conjunction with renewable energy generation, offering distinct advantages. Through continuous technological improvements and optimizations, PEM electrolysis is expected to become one of the mainstream technological routes for green hydrogen production in the future, making an important contribution to the promotion and application of clean energy.