Recently, Horizon Power's vanadium flow battery project for Kununurra has been trending across the internet. But why are vanadium flow battery projects becoming more and more prevalent? To understand this, we should start by learning more about vanadium flow batteries:

Vanadium Flow Battery: A New Era in Energy Storage
A Vanadium Flow Battery (VFB) is a type of battery in which both the positive and negative electrodes use circulating vanadium solutions as the energy storage medium. Through the process of charging and discharging, the battery enables the conversion between electrical energy and chemical energy, thereby storing and releasing energy.
The structure of a Vanadium Flow Battery is different from conventional lithium-ion batteries and lead-carbon batteries. It consists of the following key components: a stack (or individual cell), a positive electrolyte tank (storing the positive electrolyte), a negative electrolyte tank (storing the negative electrolyte), a circulating pump, and a management system. The stack is made up of multiple individual cells connected in series, each including the positive electrode, negative electrode, separator, and bipolar plates. Multiple vanadium flow battery stacks form an energy storage module, and multiple modules together constitute a complete energy storage system or station.
Principle of Energy Storage in Vanadium Flow Batteries
Vanadium ions exist in four different valence states. The active energy storage material in the positive and negative electrolytes of a Vanadium Flow Battery is vanadium ions. The charging and discharging process is based on the changes in the valence states of vanadium ions in both the positive and negative electrolytes, achieving energy storage and release.
During Charging: In the positive electrolyte, vanadium ions in the +4 valence state are oxidized to the +5 state, losing an electron and generating two hydrogen ions. In the negative electrolyte, vanadium ions in the +3 valence state gain an electron and are reduced to the +2 state, consuming one hydrogen ion.
During Discharging: In the positive electrolyte, vanadium ions in the +5 valence state are reduced to the +4 state, gaining an electron and consuming two hydrogen ions. In the negative electrolyte, vanadium ions in the +2 state are oxidized to the +3 state, releasing one hydrogen ion.
The above process shows that during charging, hydrogen ions migrate from the positive to the negative side, while during discharging, the process is reversed. The electrochemical reaction inside the battery manifests as the migration of hydrogen ions, which generates an electrical current in the external circuit.
Electrode Reactions of Vanadium Flow Batteries:
Positive Electrode: ,
Negative Electrode: ,
Overall Reaction: ,
Due to its high safety, large-scale energy storage capacity, long charge and discharge cycle life, recyclable electrolyte, cost-effectiveness throughout its lifecycle, and environmental friendliness, vanadium flow batteries (VFBs) have gained increasing global attention in recent years. Research, development, and engineering applications of VFB energy storage systems have made significant progress, with rapid development, improving technology, decreasing costs, and entering the stage of industrialization and widespread application, presenting enormous market potential.
2. Technical Features of Vanadium Flow Batteries
Technical Advantages
① Intrinsic Safety and Environmental Friendliness
Vanadium flow battery energy storage systems are intrinsically safe and reliable in operation, with an environmentally friendly lifecycle. The electrolyte in vanadium flow batteries consists of an aqueous solution of vanadium ions in dilute sulfuric acid. As long as the charge and discharge cutoff voltage is controlled properly and the battery system is stored in a well-ventilated space, it is inherently safe without the risk of fire or explosion. The electrolyte is circulated within a sealed space and does not typically produce environmental pollutants during use, nor is it contaminated by external impurities.
Additionally, both the positive and negative electrolytes in the vanadium flow battery use vanadium ions, which prevents irreversible capacity degradation from the mixing of the positive and negative electrolytes. Over years of operation, capacity degradation caused by minor side reactions and the cumulative slight mixing of the positive and negative electrolytes can be regenerated and reused through online or offline regeneration.
The stack and system are mainly composed of carbon materials, plastics, and metals. When a vanadium flow battery system is decommissioned, the metal materials can be recycled, and carbon materials and plastics can be used as fuel. Therefore, the entire lifecycle of a vanadium flow battery system is safe, has a minimal environmental load, and is very environmentally friendly.
② Independent Output Power and Energy Capacity
The output power and energy capacity of vanadium flow battery energy storage systems are independent of each other, with flexible design and installation, making them suitable for large-scale, high-capacity, and long-duration energy storage.
As shown in Figure 1, the output power of a vanadium flow battery system is determined by the size and number of the battery stacks, while the energy capacity is determined by the volume of the electrolyte. To increase the output power, the electrode area of the battery stack can be increased or the number of stacks can be increased. To increase the energy capacity, the volume of the electrolyte can be increased. This makes vanadium flow batteries particularly suitable for applications requiring large-scale, high-capacity, long-duration energy storage. The output power of vanadium flow battery systems typically ranges from hundreds of watts to hundreds of megawatts, and the energy capacity ranges from hundreds of kilowatt-hours to hundreds of megawatt-hours.
③ High Energy Conversion Efficiency, Fast Start-up, No Phase Change
The energy conversion efficiency is high, and the transition between charge and discharge states is rapid. The vanadium flow battery operates at room temperature, with the electrolyte solution circulating between the electrolyte tanks and the battery stack. During the charge and discharge processes, energy storage and release occur through the changes in the valence state of vanadium ions dissolved in the aqueous solution, without any phase change.
Thus, the transition between charge and discharge states is quick, with the energy storage system in megawatt-scale energy storage able to switch from 80% charge to 80% discharge in less than 100 milliseconds, primarily determined by the transmission speed of control signals. This allows vanadium flow batteries to be used for amplitude modulation and frequency modulation, renewable energy grid integration, ancillary services, peak shaving for the power grid, and emergency backup energy storage.
④ Modular Design Facilitates System Integration and Scaling
The vanadium flow battery stack is assembled from multiple single cells stacked in a filter-press manner. Currently, the rated output power of an industrialized single cell stack is generally between 30 and 80 kW. The energy storage system typically consists of multiple modular units, each with a rated output power of around 500 kW. Compared to other batteries, vanadium flow battery stacks and energy storage system modules have large rated output power, good uniformity, and are easier to integrate and scale up.
2. Limitations of Vanadium Flow Batteries
① System Complexity
The energy storage system is composed of multiple subsystems, making it complex.
② Energy Support Equipment
To ensure continuous stable operation, the energy storage system requires additional equipment such as electrolyte circulation pumps, electronic control devices, ventilation systems, and electrolyte temperature control systems, which in turn need to be powered. As a result, vanadium flow battery systems are generally not suitable for small-scale energy storage systems.
③ Lower Energy Density
Due to the limitations of vanadium ion solubility and other factors, vanadium flow batteries have lower energy density. They are more suited for fixed energy storage stations where volume and weight are not significant constraints but are not suitable for use as mobile power sources or for dynamic batteries.
3. Life Cycle Cost Analysis of Vanadium Flow Batteries
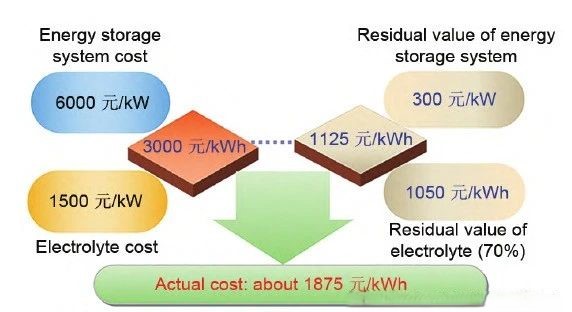
The following diagram illustrates the estimated life cycle costs of vanadium flow battery energy storage systems with 4-hour and 10-hour storage durations.
① 1 MW/10 MWh Vanadium Flow Battery Energy Storage System Actual Cost Estimate:
② 1 MW/10 MWh Vanadium Flow Battery Energy Storage System Actual Cost Estimate:
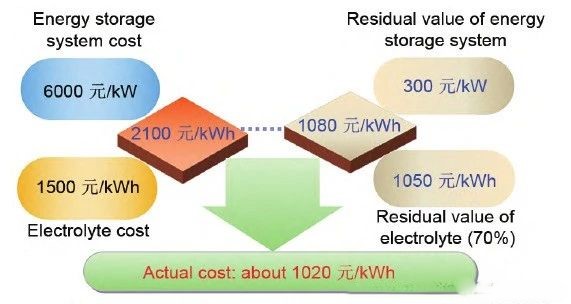
Therefore, for vanadium flow battery energy storage systems, the longer the energy storage duration, the lower the overall lifecycle cost.
4. Industry Chain Composition
The vanadium flow battery industry chain includes upstream materials, battery manufacturing, module design, and system integration. The mainstream liquid flow battery currently being researched is the vanadium flow battery. Its upstream raw materials primarily include vanadium pentoxide (V2O5) and perfluorosulfonic acid membranes. The midstream involves the design and manufacturing of vanadium flow battery storage systems, which consist of components such as inverters, smart controllers, fuel stacks, membranes, electrolyte, and storage tanks. Among these, the most critical components are the fuel stack and electrolyte. The downstream applications include wind power generation, photovoltaic power generation, grid peak-shaving, and more.
Vanadium Ore and Vanadium Processing
Vanadium is a lithophile element, typically found in a dispersed state in ores. Its natural distribution characteristics are large reserves, widespread distribution, and low content. Vanadium-titanium magnetite is the most common vanadium-bearing ore. This mineral is found globally and is currently the primary source of vanadium, accounting for over 85% of global annual vanadium production.