The Oxygen Evolution Reaction (OER) plays a crucial role in various clean energy storage and conversion processes, including Water Electrolysis and metal-air batteries. In PEM Water Electrolysis for hydrogen production, OER is the core step that takes place at the anode, where water molecules are oxidized to produce oxygen. The principle behind this reaction can be broken down into the following key points:
1. Basic Reaction Mechanism
In the Water Electrolysis process, OER occurs at the anode (positive electrode) via a multi-electron transfer mechanism, leading to the oxidation of water. The specific reaction path depends on the electrolyte environment:
Alkaline Conditions (e.g., KOH or NaOH solution):
Anode reaction: 4OH⁻ → 2H₂O + O₂↑ + 4e⁻
Overall reaction: 2H₂O → 2H₂↑ + O₂↑
The process involves four consecutive electron transfer steps, with intermediates (OHₐdₛ→Oₐdₛ→OOHₐdₛ→O₂ₐdₛ) undergoing adsorption and desorption.
Acidic Conditions (e.g., H₂SO₄ solution):
Anode reaction: 2H₂O → 4H⁺ + O₂↑ + 4e⁻
Water molecules lose electrons directly to generate oxygen and protons.
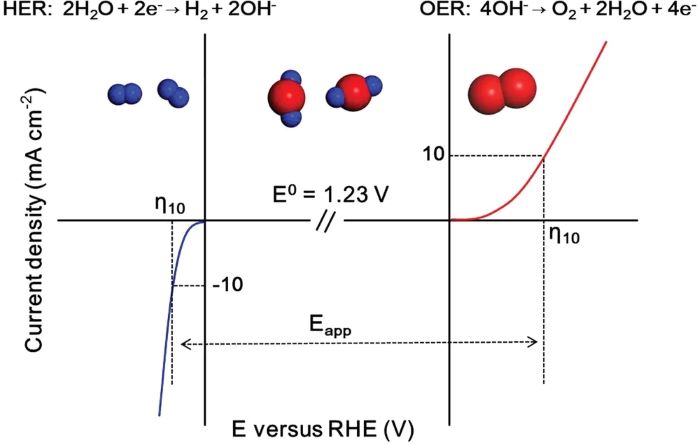
2. Kinetic Challenges and Overpotentials
OER is a four-electron reaction, which has high activation energy and slow kinetics, leading to the need for high overpotentials (typically above 300 mV) to drive the reaction. For example:
Adsorption-Driven Evolution Mechanism (AEM): In traditional pathways, differences in the adsorption free energy of intermediates lead to the rate-determining step (e.g., formation of OOHₐdₛ), creating a bottleneck.
Lattice Oxygen Evolution Mechanism (LOM): Some catalysts directly involve lattice oxygen in the reaction, bypassing intermediate formation, which can reduce the energy barrier but may compromise stability.
3. Comparison of OER in PEM Water Electrolysis, ALK, and AEM Electrolyzers
1. Reaction Environment and Mechanism:
PEM Water Electrolyzers:
Environment: Acidic (pH ≈ 0-2), with pure water as the reactant at the anode.
Mechanism: Water molecules are oxidized at the anode to produce oxygen, with the reaction pathway: H₂O → OH∗ → O∗ → OOH∗ → O₂ (involving 4-electron transfer).
Challenge: High corrosiveness of materials in acidic environments, and the slow OER kinetics requiring high overpotentials to drive the reaction.
ALK Electrolyzers:
Environment: Strongly alkaline (pH ≈ 14), with a 30% KOH solution as the electrolyte.
Mechanism: Hydroxide ions (OH⁻) are oxidized at the anode to produce oxygen: 4OH⁻ → O₂ + 2H₂O + 4e⁻.
Challenge: High intermediate adsorption energies in alkaline environments lead to higher overpotentials.
AEM Electrolyzers:
Environment: Neutral or weak alkaline (pH ≈ 7-10), with pure water or low-concentration alkaline solutions.
Mechanism: Similar to ALK, but with Proton Exchange Membranes for Electrolysis conducting OH⁻ ions, and the reaction pathway is similar to alkaline electrolyzers.
Advantages: Combines the low cost of ALK with the high current density potential of PEM.
2. Reaction Kinetics and Overpotentials:
PEM Water Electrolyzers: OER is the main bottleneck of the system, with overpotentials contributing up to 60%-70% of the total energy requirement.
ALK Electrolyzers: OER overpotentials are relatively high, but can be mitigated through non-precious metal catalysts.
AEM Electrolyzers: OER kinetics lie between PEM and ALK, and optimizing the combination of catalysts and membranes is crucial.
4. Anode Catalysts in PEM Water Electrolysis, ALK, and AEM Electrolyzers
1. PEM Water Electrolyzers:
Catalysts: Primarily iridium-based oxides (IrO₂), which offer high catalytic activity and stability in acidic environments.
Challenges: Iridium is scarce and expensive (accounting for over 40% of the electrolyzer cost), requiring low-iridium strategies (such as core-shell structures and alloying) to reduce costs.
Alternatives: Exploration of ruthenium-based (RuO₂) catalysts, although their stability under acidic conditions is limited.
2. ALK Electrolyzers:
Catalysts: Nickel-based materials like NiO and Ni-Fe layered double hydroxides (LDH), which are low-cost and stable in alkaline environments.
Optimization: Doping (e.g., with Fe, Co) to enhance activity or using porous structures to increase surface area.
3. AEM Electrolyzers:
Catalysts: Transition metal oxides (e.g., Ni-Fe oxides, Co-based materials), balancing cost and adaptability to alkaline/neutral environments.
Advantages: No need for precious metals, but the interface stability between catalysts and Proton Exchange Membranes for Electroplating needs to be addressed.
Conclusion:
The OER reaction mechanism and catalyst selection in different electrolyzers are directly influenced by the environment and material costs. In the future, balancing activity, stability, and cost will be essential to drive the large-scale adoption of green hydrogen technologies. Proton Exchange Membranes for Electrolysis are essential components for the efficient and cost-effective production of hydrogen in electrolysis systems.