Proton exchange membrane (PEM) fuel cells are among the most promising technologies for achieving "carbon peak" and "carbon neutrality." Although PEM fuel cells have experienced ups and downs over the past few centuries, they currently play a crucial role in building a sustainable society. Today's PEM fuel cells offer significantly lower platinum (Pt) loadings compared to earlier generations. For instance, the total Pt loading of the first-generation Toyota Mirai fuel cell (2017), which was the first commercialized PEM fuel cell vehicle, is only 0.365 mg cm⁻², a substantial reduction compared to the first practical fuel cell from 1962, which had a Pt loading of 35 mg cm⁻² and used potassium hydroxide solution as the electrolyte. The significant advancements in PEM fuel cells are attributed not only to the development of catalytic layers but also to the replacement of traditional acid/base solution electrolytes with advanced perfluorosulfonic acid resins (such as Nafion). Since their introduction in the 1970s, these materials have evolved the structure of membrane electrode assemblies (MEAs) and related manufacturing processes.
PEM fuel cells have gradually found commercial applications, such as serving as power sources for vehicles. Companies like Toyota, Hyundai, and Honda have launched fuel cell vehicles on the market. However, PEM fuel cells currently face competition from internal combustion engines and batteries, primarily due to their high costs and shorter lifespans. To overcome these challenges, the development of advanced materials and manufacturing technologies is essential. This progress requires close collaboration between businesses, universities, research institutions, customers, and governments. In this process, fundamental research should focus on developing high-performance and durable MEAs, while industrial efforts should consider scaling up the production of key materials and components. Currently, the components of MEAs, including catalysts, ionomers, membranes, and gas diffusion layers (GDLs), have been successfully implemented in industrial production. However, integrating these materials into MEAs often results in significant performance losses. The technical community has placed considerable attention on the compatibility of components and has developed improved MEA manufacturing processes based on this understanding.
2. Latest Advances in Key Materials for Membrane Electrode
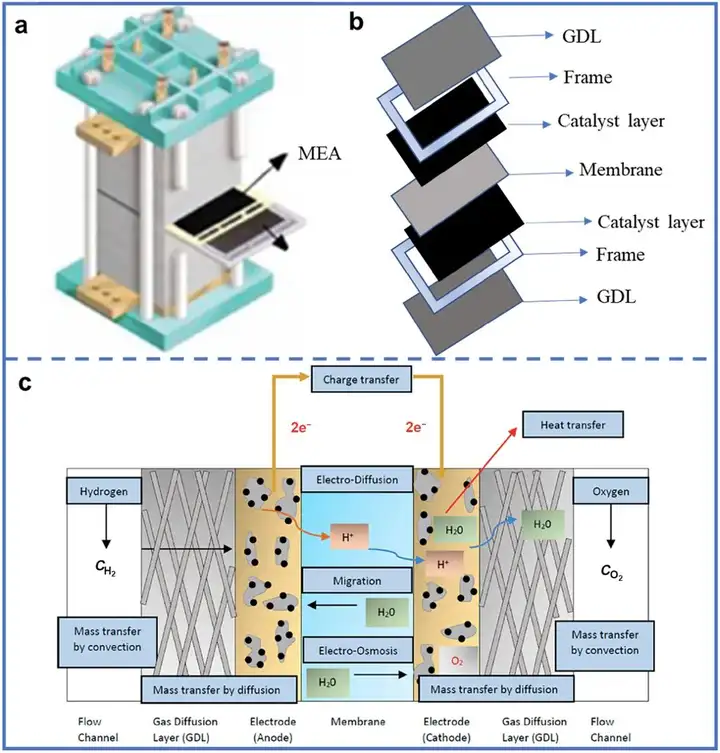
The MEA is the main site for electrochemical reactions and plays a core role in PEM fuel cells. MEAs typically consist of six main components: catalysts, ionomers, proton exchange membranes, gas diffusion layers (GDLs), adhesives, and frames. The operating mechanism of MEAs is illustrated in the figures. Electrical energy is generated through independent redox reactions occurring at the anode and cathode. Therefore, studying the kinetics of these redox reactions is essential, which requires efficient catalysts to accelerate the reaction kinetics. Typically, catalysts operate in the catalyst layer, located between the GDL and the PEM. To facilitate proton transfer in the catalyst layer and enhance its mechanical strength, ionomers with proton-conducting properties need to be applied. The composition of the ionomer usually matches that of the proton exchange membrane, allowing rapid proton transfer from the anode to the cathode while preventing crossover of hydrogen and oxygen during operation. Additionally, the hydrophobic GDLs on both sides are crucial for gas distribution and removing excess moisture, which is essential for water management in fuel cells. These materials are at the core of MEAs.