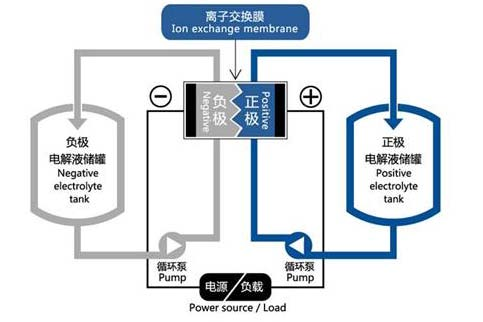
A flow battery is a type of rechargeable battery system that consists of several essential components: a stack, electrolytes, electrolyte storage tanks, circulation pumps, pipelines, auxiliary equipment, and monitoring and protection devices. Since flow batteries involve strong oxidative and reductive properties, all components must be made of corrosion-resistant materials, typically plastics or anti-corrosion linings.
Electrolyte storage tanks are used to hold the electrolytes and are usually made from materials such as PP, PVC, or PE. The safety and reliability of these tanks are critical because any leakage could lead to electrolyte loss and severe environmental pollution.
The circulation pump drives the flow of electrolytes, ensuring continuous circulation through the stack for charging and discharging. If the pump fails, the entire flow battery system halts operation. This makes the pump’s reliability crucial. Common pumps include PP plastic pumps and PTFE pumps, with popular types being centrifugal pumps and magnetic pumps.
Auxiliary equipment includes filters, flow meters, pressure sensors, and heat exchangers. Among these, heat exchangers play a vital role. Unlike other energy storage systems, flow batteries dissipate heat through electrolytes, which carry it away from the stack. Using cooling mediums, the system can easily regulate its temperature, making temperature control simple and one of the reasons why flow batteries are suitable for large-scale energy storage applications. Heat exchangers are typically water-cooled or air-cooled, using materials like PP, PE, or PTFE.
The Role of Ion-Conductive Membranes in VRFBs
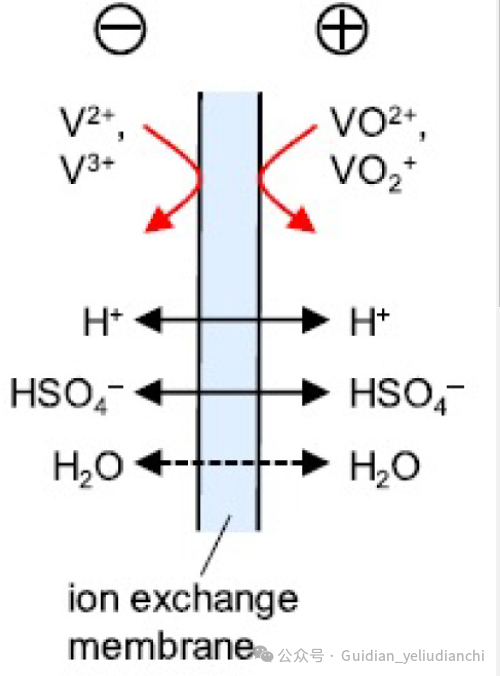
Ion Exchange membranes are critical components in vanadium redox flow batteries (VRFBs). These membranes must allow protons to pass while minimizing the crossover of vanadium ions in different oxidation states, reducing self-discharge and improving the battery's coulombic efficiency.
An ideal membrane should have low resistance and excellent conductivity to reduce ohmic losses and should also exhibit chemical stability to enhance the battery’s cycle life. The membrane’s performance directly affects efficiency, capacity, and overall battery durability.
Key characteristics of an efficient ion-conductive membrane include:
High proton conductivity.
Low permeability to vanadium ions and water molecules.
Superior chemical durability.
Adequate mechanical strength for long-term operation.
Advances in Membrane Materials
In the current research and application landscape, perfluorosulfonic acid membranes such as Nafion, developed by DuPont, are widely used due to their excellent performance. However, their high cost limits broader adoption. A cost-effective alternative is the ProtoneX membrane, manufactured by China’s Ging Hope. ProtoneX offers performance comparable to Nafion and has gained recognition in the energy storage industry.
Researchers continue to explore new membrane materials that reduce costs while maintaining performance. However, many alternatives still face challenges in terms of chemical stability, vanadium ion selectivity, and mechanical strength. Bridging the gap between laboratory research and real-world application remains a key focus for advancing flow battery technology.
By combining these innovations with the inherent advantages of flow batteries, such as scalability and efficient thermal management, this technology is poised to play a significant role in the future of renewable energy storage.